Near-infrared,photocatalysis,based,on,upconversion,nanomaterials
时间:2023-01-22 09:55:06 来源:天一资源网 本文已影响 人 
Xingyuan Guo(郭星原) Zhe Wang(王哲) Shengyan Yin(尹升燕) and Weiping Qin(秦伟平)
1College of Physics,Jilin University,Changchun 130012,China
2State Key Laboratory on Integrated Optoelectronics,College of Electronic Science and Engineering,Jilin University,Changchun 130012,China
Keywords: near infrared,photocatalysis,upconversion,semiconductor
As the global energy crisis and environmental pollution continue to worsen, developing environmentally friendly technologies is imperative. Among these new technologies,semiconductor-based photocatalysis is a good candidate for contaminant remediation because it can utilize both solar energy and artificial light sources. In chemistry, the photocatalytic reaction involves the modification of the photoreaction rate by adding catalysts.[1]Taking semiconductor materials as an example, when a light (with photon energy equal to or greater than the forbidden band width of the semiconductor,hν ≥Eg) irradiating the semiconductor, the electrons in the valence band transport to the conduction band to yield photogenerated electrons, and photogenerated holes are simultaneously generated in the corresponding position in the valence band. The separated electrons and holes have reducing and oxidizing properties, respectively. The photogenerated electrons and holes can migrate to the surface of the materials,and then, through the reduction or oxidation reaction, some photoreactions occur, such as water splitting, CO2reduction,and pollutant degradation.[2–5]For the convenience of later discussion,we divide semiconductors into narrow-band semiconductors (Eg<1.65 eV or wavelengthλ >750 nm), wide semiconductors (Eg>3.1 eV orλ <400 nm), and visible light-responsive semiconductors (1.65 eV<Eg<3.1 eV or 400 nm<λ <750 nm).
The wide bandgap of some semiconductors such as TiO2and ZnO has been a key restriction for effective solar energy usage.[6,7]The energy proportion of ultraviolet (UV) light in the solar spectrum is only 5% and thus quite low in comparison with visible (Vis) (~48%) and infrared (IR) (~44%)light. The poor usage of sunlight has hampered the photocatalytic efficiency of pure TiO2in pollutant removal applications. To promote solar energy utilization in semiconductor photocatalysis, several approaches such as semiconductor coupling and impurity doping[8,9]have been adopted to modify the bandgap of semiconductors and extend their absorption region to the Vis range.[10]Another approach to solving this problem is exploiting the surface plasmon resonance of noble metals.By controlling the size and morphology of noble metal nanoparticles(NPs)on the semiconductor surface,the absorption of the semiconductor can be red-shifted to the near-IR(NIR) region.[11]However, the intrinsic photocorrosion susceptibility and high processing expenses reduce the viability of this solution. With the development of upconversion NPs(UCNPs),the scope of their utility has been greatly improved despite their reported inefficiency in some applications, and it seems practical to couple semiconductors with UCNPs that can utilize abundant NIR light,improve the availability of solar energy,and ultimately strengthen photoreactions.[12,13]
In 2010, our group reported for the first time that the broadband semiconductor TiO2could be activated by NIR energy, which was a significant milestone for NIR photocatalysis.[14]NIR-to-UV UCNPs emit UV light after absorbing NIR light, and TiO2then generates strongly oxidative holes (h+) and reductive electrons (e-) under UV light excitation. Using this strategy, novel NIRresponsive photocatalysts includingβ-NaYF4:Yb,Tm@ZnO nanocomposites,[15,16]NaYF4:Yb,Tm@TiO2core–shell NPs,[17]NaYF4:Yb,Tm/CdS/TiO2composites,[18]and NaYF4:Yb,Er/CdSe composites,[19]have been developed,and the energy transfer(ET)between rare earth ions and the semiconductor has been studied using steady-state and dynamic fluorescence spectroscopy. The results have demonstrated that the F¨orster resonance energy transfer (FRET) between rare earth ions and the semiconductor is a key factor affecting the utilization efficiency of NIR light. In this paper, NIR photocatalysts of UC/semiconductor composites are reviewed including the different types of photocatalysts based on rare earth ions doped in the nanomaterials.
UC is an optical process that has the distinctive property of absorbing two or multiple long-wavelength photons in sequence and radiating out shorter wavelength photons;in other words, this process converts lower-energy photons to higherenergy photons because of the luminescence originating from transitions between electrons in the 4f or 4f–5d shell. A narrow spectral line and pure chromaticity have been obtained since the mid-1960s, and these characteristics have been intensively investigated and widely used in optical devices.With the rapid development of nanotechnology over the past decade,high-quality rare earth-doped UCNPs have been successfully prepared and improved in many areas, especially in biomedical applications such as biodetection,bioimaging,drug delivery,photodynamic therapy,photothermal therapy,and disease therapy.[20–26]
2.1. UC mechanism
Research on the mechanism of UC luminescence(UCL)has mainly focused on the mechanism by which rare earth ions achieve energy level transitions. The explanation of the luminescence principle was developed with the emergence of new materials,and the transition mechanism differs because of the different host materials and activated ions. The main luminescence mechanism is illustrated in Fig.1.

Fig.1. Principal diagrams for the UC processes of Ln3+-doped crystals. (a)Excited-state absorption(ESA),(b)successive energy transfer(SET),(c)cross relaxation(CR),(d)cooperative UC(CU),(e)photon avalanche(PA),and(f)energy migration-mediated UC(EMU).The red,green,and purple lines indicate photon excitation,energy transfer,and emission processes,respectively.
2.1.1. Excited-state absorption and successive energy transfer
The foundational principle of excited-state absorption(ESA)is a process in which the electron of a single ion jumps to the excited state energy level (E1or E2) from the ground state energy level (G) through the continuous absorption of multiphotons(Fig.1(a)). The electrons in the excited state of sensitizers have a high chance of reaching a higher excited state in the successive absorption process, which has been termed the Auzel APTE effect.[27]If the number of electrons at this energy level is sufficiently large to induce population inversion,UC laser emission can be achieved.Similarly,successive energy transfer (SET) typically occurs between different types of ions, as shown in Fig. 1(b). When used as sensitizers(S),Yb ions can strongly absorb photons because of their large absorption cross-section in the NIR region, and Pr, Nd,Eu,Gd,Tb,Dy,Ho,Er,and Tm can be used as activators(A).Energy transfer from the sensitizer to the activator in such a system populates both emission and intermediate levels. The transfer process distinguishes radiative transfer, nonradiative,and multi-phonon-assisted ET.The probability of nonradiative ET is strongly dependent on the S–A distance.[27]When the doping concentration of rare earth ions is high, the average distance between ions decreases, and the probability of nonradiative ET between ions increases. When there is an energy mismatch between the activator and sensitizer,multi-phonons assist in converting the extra energy during ET into lattice vibration energy or to carry out ET.[28]
2.1.2. Cross relaxation
Cross relaxation(CR)can occur between ions of the same and of different types. When enough ions are excited to an intermediate state, the excited-state ions may couple through nonradiative transitions,one returning to the ground state or a lower intermediate state,the other transitioning to a higher energy level. Then, they generate a radiative transition.[29]The luminescence of doubly doped ionic materials falls under this category.
2.1.3. Cooperative UC
Cooperative UC (CUC) includes two processes: cooperative luminescence (CL) and cooperative sensitized luminescence (CSL).[30,31]CL involves the interaction of the same ions in the excited state to form a virtual energy level and realize UC luminescence when returning from the virtual energy level to the ground state. The CL of three ions was observed in the Yb3+-doped CaF2matrix under NIR excitation.[32]The CSL realizes the energy of the virtual level to the other ions to generate that of the CUC.In Ref.[23],the cooperative sensitization of one Gd3+ion or one Pb2+ion by four or three excited Yb3+ions was demonstrated experimentally.[32–34]
2.1.4. Photon avalanche
Photon avalanche (PA) combines the processes of excited-state absorption and ET.[35]When the doping concentration of rare earth ions is sufficiently high, an obvious PA process occurs,and the pump photons continue to be absorbed by ions in the excited state energy level E.Then,the ions transition to the excited state energy level E2, resulting in a large increase in the number of ions populated on E2. When the population of ions reaches a certain number, a strong radiation transition occurs at the E2energy level, that is, the PA phenomenon.[35]
2.1.5. Energy migration-mediated UC
Researchers have utilized a sensitizer (Yb3+) that can capture pump photons and promote the excited states of a nearby accumulator ion (Tm3+) in a core–shell structure.[36]The resultant energy is subsequently transmitted to a migrator (Gd3+) from the accumulator’s high-lying energy states.Ultimately, the capture of migrating energy by activated ions is achieved via random energy hopping through the migratory ion sublattice. Significantly,researchers were able to produce effective UC emissions at room temperature with modest excitation densities.[37]
It is noteworthy that different rare earth ions generally have different UC luminescence modes,and the same ion has different luminescence mechanisms under different pumping modes.
2.2. Methods for improving UCL efficiency
Despite extensive research, the low quantum yield (QY)of UCNPs under NIR light remains a fundamental barrier to their widespread application. Therefore,different methods for improving their efficiency have been developed,[38–40]which can be categorized into the following categories.
2.2.1. Suitable matrix material
Owing to the influence of ET between dopant ions and acceptor ions,the key to increasing the UC efficiency of Ln3+is the structure of the matrix materials. Because the local site symmetry, crystal field strength, and phonon energy of the host materials affect the ET efficiency between rare earth ions,these factors provide different probabilities of transition in the f–f shell of the Ln3+ions, leading to tunable optical properties of UC materials. The most extensively studied host matrices with low phonon energies are fluorides (~355 cm-1),chlorides (~260 cm-1), iodides (~144 cm-1), and bromides(~172 cm-1). The efficiency of UC luminescence for these matrices is as follows:fluorides<chlorides<iodides<bromides;their structural stability decreases in the inverse order. Among these fluorides,β-NaYF4has been acknowledged as the most efficient UC host material in the past decades because of the low symmetry of the Ln3+-doped hexagonal structure,which leads to a UC efficiency up to ten times higher than that of its cubic phase.[41]Currently, certain novel UC host materials,such asβ-NaLuF4and LiLuF4,have been discovered to provide a higher UCL output yield thanβ-NaYF4.The phonon energy of the host matrix has a significant impact on the UC efficiency.[42]Host materials with low phonon energies are advantageous for Ln3+doping to generate intense UCL,because the low phonon energy facilitates energy mismatching during ET.[43]The UC efficiency relies on the phonon energy of various host lattices, and phonon-assisted ET is key to enhancing UC emission intensity.[44,45]
2.2.2. Optimizing the A–S concentration ratio
Optimization of the dopant concentration in a single NP is widely considered to be the simplest technique for increasing UC QY. The underlying mechanism of this technique relies on controlling the distance-dependent ET and CR as well as the concentration-dependent photon absorption. Concentration quenching, a typical nonradiative process, can be prevented by optimizing dopant concentrations. At high doping concentrations, this process often occurs in certain Ln3+activators with rich energy levels (e.g., Tm3+, Er3+, Nd3+,and Pr3+) via a CR process, leading to nonradiative depopulation of the excited activator ions. Consequently, the UC QY and lifetime decrease as dopant concentration increases(typically activator ion mole ratios below 2 mol%). In some hosts, concentration quenching may be relieved by majorization of synthesis and intensifying the excitation power density.Recently, Jinet al.demonstrated that high excitation irradiance (2.5×106W/cm2) was able to alleviate concentration quenching when evaluating UCL.At the same time,when the activator concentration increased,for example,when Tm3+in NaYF4increased from 0.5 mol%to 8 mol%, the UCL signal increased remarkably up to a factor of 70.[46]
2.2.3. Appropriate cation incorporation
Doping appropriate Ln3+or non-Ln3+ions into the matrix of colloidal NPs is an important strategy for controlling the size, shape, electronic, magnetic properties, and UCL intensity of UCNPs.[47,48]By incorporating these ions, the ET redistribution between the emitting levels of the emitter, local site symmetry, and crystal field affects the luminous efficiency of rare earth ions.[49–51]Incorporated ions can act as both sensitizers and bridges for ET.The ET processes involved also vary. For example, emission QY increases ten-fold with Li+and Er3+co-doped Y2O3NPs.[52]Gd3+and Nd3+ions have been reported to serve as energy migration ions to facilitate the UCL of emitters via the EMU process.[53]In addition,some studies have shown that doping Mn2+,[54]Ca2+,[55]Zn2+,[56]and Sn2+[57]into the lattice can also significantly improve UCL intensity.
2.2.4. Surface passivation
The large specific surface area of UCNPs usually leads to severe nonradiative transitions,which are the main cause of the low UC QY of Ln3+-doped matrices.[58,59]To overcome these deficiencies, surface passivation is considered an efficient method for minimizing the influence of surface states and enhancing solar cell efficiency.[60,61]For UCNPs, the smaller the size, the more surface defects, which causes significant quenching of the luminescent center. Coating the surface with a sufficiently thick shell layer can effectively reduce luminescent quenching and improve luminescent performance. Core–shell materials are divided into homogeneous and heterogeneous coatings. Homogeneous cladding utilizes the same host material and the core can be regarded as a seed crystal. The cladding process can be regarded as epitaxial generation,and a layer-wise heterostructure can be achieved. Furthermore,predesigned energy exchange can be realized for various types of dopant ions,which plays an important role.[62,63]A heterogeneous core–shell structure uses a completely different compound but with similar lattice constants, and UCL enhancement can also be obtained. For example,the UCL strength of the as-preparedα-NaYF4: Yb,Er@CaF2core–shell NPs was~300 times stronger than that of the uncoated UC sample.[64]Compared to the aforementioned methods,there is significant potential in exploiting the synergistic effects of one-half engineered NIR dyes to construct efficient UC systems.[65]
2.2.5. Plasmonic enhancement
Surface plasmon resonance(SPR)refers to the resonance of free electrons on a metal surface when incident light hits the interface of two media with different refractive indices (such as gold or silver coating on a glass surface) at a critical angle. At a nanoscale sample size,wave propagation is limited.Surface plasmons are restricted to nanoparticles that are comparable in size or smaller than the wavelength of light used to excite plasmons, which is referred to as localized surface plasmon resonance (LSPR).[66,67]In general, the wavelength of the SPR is placed between the excitation and the emission wavelength center. During the fluorescence emission of the emitter, SPR greatly affects spontaneous radiation transition in the range of the local field. When the distance between noble metal NPs and the UCNPs is suitable,the quenching effect of SPR on UCNPs is suppressed,and the rate of radiative transition increases at the same time,which manifests as a significant decrease in the fluorescence lifetime. This process is referred to as the Purcell effect.[68]The coupling of UCNPs with noble metal NPs results in an improved UC intensity.[69,70]Because UCL is dependent on SPR frequency,the size and shape of noble metal NPs can adjust the SPR from Vis to IR bands and then adjust the emission intensity of rare earth ions in different bands.[66]
In addition to these factors, the purity of rare earth materials significantly influences the UCL.The purity of the raw materials for synthesizing UC materials should reach 5N–6N.When the purity of rare earth materials is insufficient,the lowpurity rare earth element will show a characteristic peak in the spectrum, which can easily cause a change in the UCL spectrum due to the incorporation of other rare earth elements and affect A–S luminescence.[71]
To extend the absorption region of semiconductors,semiconductor coupling and impurity doping have been used to alter band structure.[72]Utilizing UC on these materials results in increased efficiency in terms of photocatalytic performance of classic UV or Vis active photocatalysts. This occurs because the light absorption range is successfully expanded to the IR region,which accounts for 44%of solar energy.
3.1. Development of new narrow-bandgap semiconductor materials with NIR light response
Narrow-bandgap semiconductors can harvest ultraviolet,Vis, and NIR wavelengths of the solar spectrum. These materials have significant potential for utilizing solar energy in the immediate future.[73]Identifying semiconductors that can respond to the full solar spectrum is challenging. Shiet al.realized efficient decomposition of water for hydrogen production using a wavelength greater than 900 nm through a molecular-semiconductor photocatalytic system constructed using inexpensive nickel phytic acid nickel and polymeric carbon nitride.[74]Organic dye molecules were degraded by W18O49/N-doped reduced graphene oxide hybrid nanomaterials under NIR light irradiation.[75]Liuet al.discovered that WS2and Bi2WO6nanosheets had NIR photocatalytic properties for the first time and verified the photocatalytic process under the action of NIR light using various research methods. They also confirmed that a narrow-bandgap semiconductor material with an appropriate energy level structure could absorb NIR light,generate carrier separation,and form certain free radicals, thereby realizing the degradation of organic molecules.[73,76]Huanget al.synthesized a new lowdimensional nanocrystalline material Cu2(OH)PO4by a hydrothermal method. Under NIR irradiation, it exerted a photocatalytic degradation effect on some organic compounds.[77]In addition, for narrow-band semiconductor materials, when the photon energy exceeds the forbidden bandwidth, the remaining photon energy after excitation is converted into heat energy in the semiconductor, resulting in significant thermal loss. Thermal energy will seriously affect the interval between photogenerated electrons and holes;at the same time,the narrow band gap increases the probability of reorganization of the photogenerated electrons and holes.
3.2. Direct doping of rare earth ions into semiconductor materials to prepare NIR photoresponsivity materials
In recent studies,bismuth vanadate(BiVO4)has attracted significant attention because of its abundance,nontoxicity,and excellent Vis-driven catalytic performance as a matrix material. Many strategies, such as doping, heterojunction, and defect engineering, have been employed to achieve higher BiVO4photocatalytic efficiency.[78]Col´onet al.achieved NIR photocatalysis using Er3+ions single-doping or Yb3+and Er3+co-doping of BiVO4.[79,80]On this basis, researchers have further explored the practical applications of this new material,such as improving the efficiency of NIR light utilization in solar cells.[81,82]In particular,perovskite,a target of significant research interest,has achieved a high photoluminescence QY by doping with different Ln3+ions(Ln3+=Er3+,Ce3+,Sm3+, Eu3+, Tb3+, Dy3+, and Yb3+) and multicolor emission from Vis to NIR regions.[83]Compared with Vis and ultraviolet light, NIR light is characterized by low absorption,minimal scattering, high transmittance, and low damage to biological tissues. NIR light-responsive semiconductor materials can be used for sustained drug release and photodynamic therapy.[84,85]Although the response to NIR light can be achieved by doping semiconductors with rare earth ions,its utilization efficiency remains questionable. It is well known that the UCL capacities of rare earth ions and host materials are key factors influencing the resulting UCL of rare earth ions. Recent research results show that rare earth fluoride materials remain useful for achieving high-efficiency UCL.
3.3. Preparation of novel NIR light-responsive semiconductor composites using UC and semiconductor materials
Using a UC material as the core and a semiconductor as the shell,composite materials have constructed via core–shell preparation technology. This heterostructure can fully utilize the excellent light-to-frequency conversion performance of UC to effectively excite semiconductor materials. Following this approach, ZnO@UC and TiO2@UC effectively degraded target pollutants under NIR irradiation.[15,17]When the heterojunction of CdS and TiO2induced adhesion on the NaYF4:Yb,Tm microcrystal surfaces, the individual adsorption of CdS or TiO2on the NaYF4:Yb,Tm microcrystal surfaces demonstrated substantially lower catalytic activity than the composite.[18,86]These consequences show that the heterojunction structure has a significant impact on the separation of photogenerated electrons and holes. In ZnO NIR photocatalytic materials,it was found thatα-NaYF4andβ-NaYF4NPs were the same size as the same doping contents (Yb,Tm) under the same reaction conditions. After compounding with ZnO, the NIR photocatalytic efficiencies were similar. It is well-known that the fluorescence efficiency ofβ-NaYF4is much higher than that ofα-NaYF4. We studied the surface structure of the compounded ZnO material and found that the phase structure of NaYF4would affect the surface crystallinity of the semiconductor and led to different catalytic effects.[16]Therefore,the photocatalytic activities of NaYF4:Yb and Er/CdSe composites were evaluated using methyl blue under 1560 nm irradiation.[19]Upon 1560 nm excitation, the energy levels2H9/2,2H11/2,4S3/2, and4F9/2of the Er3+ions were populated, and this excited state lifetime decreased when CdSe NPs were attached to the NaYF4:Yb,Er microrods. Compared to the efficiency of ET and the decay of emission intensity,these values suggest that FRET and photon reabsorption ET processes exist.
4.1. Photocatalysis
Photocatalysis has attracted significant attention since TiO2has been used for the photoelectrical catalysis of hydrogen generated from water.[7]To date, applying composite preparation and modification techniques to traditional semiconductor materials have facilitated the full utilization of the solar light spectrum and should be applied in the field of environmental protection. NIR catalysis has been investigated in a variety of environmental applications,including environmental remediation,water splitting,and CO2reduction.[87–89]In our previous report,[19]as shown in Fig.2, Yb3+/Er3+codoped NaYF4microrods have efficient NIR-to-Vis UC agents and strong emission peaks (379 nm, 408 nm, 520 nm, and 539 nm) after being excited by light at 1560 nm; therefore,they are appropriate candidates for NIR photocatalysis. Moreover, the NaYF4:Yb3+, Er3+/CdSe core-shell structure was chosen because it was conducive to the absorption of UV or Vis light from the UC microrods. The time-resolved fluorescence decay of rare earth ions was measured to analyze the ET process between rare earth ions and the semiconductor. FRET and radiation reabsorption are two ET processes in the catalytic process based on the lifetime of the excited state of the luminescence centers.Furthermore,the FRET process is more efficient because of the ET from the excited states of Ln3+ions to the semiconductor directly. Although significant progress has been made in NIR-active photocatalysts, there are many obstacles to commercialization.
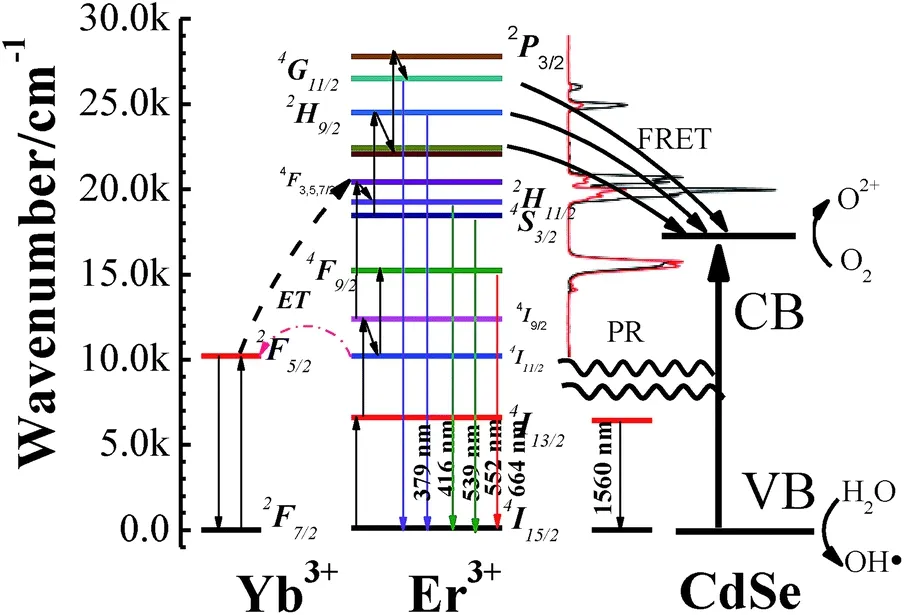
Fig. 2. Diagrams of ET between semiconductor and UC (Reprinted with permission from Copyright(2016)Royal Society of Chemistry).[19]
4.2. Biological applications
Biological tissues are only transparent in the NIR window (700 nm–1000 nm) because light absorption and scattering by biomolecules in the tissue is lowest in this spectrum. If longer-wavelength light sources such as IR light can be employed for photoactivation,this would be highly beneficial for biological applications. The most commonly used excitation sources for biomedical photoactivation applications,such as photodynamic therapy[90]and photo-triggered drug release,[84]are Vis and UV light, mainly because the photosensitive compounds employed in these techniques are sensitive to these wavelengths. UV and Vis light penetrate tissues poorly, and UV light is particularly harmful to the human body. Thus, NIR-responsive semiconductor composites can be used to overcome these limitations. Multifunctional nanocapsules, doxorubicin (DOX, an antineoplastic drug)-loaded UCNPs@SnO2-bovine serum albumin (BSA), were prepared using different preparation processes, as shown in Fig. 3.[91]UCNPs@SnO2acted as the UCNP shell, and the surface mesopores were used to load drugs. When NIR excites UCNPs, Nd3+-doped UCNPs generate heat to achieve the phototherapy effect,and DOX triggers chemotherapy. UV or Vis photosensitization of SnO2produced reactive oxygen species by Tm3+-doped UCNPS, which further enables photodynamic therapy to ablate tumor cells. Moreover, different elements play a role in fluorescence imaging. For example,Sn can be used as a contrast agent in computed tomography to monitor the treatment process. Gd3+ions exhibit strong paramagnetism and can be used for magnetic resonance imaging.

Fig.3.(a)Fabrication process of UCNPs@SnO2-DOX/BSA nanocapsules(DOX,an antineoplastic drug).(b)Schematic representation of nanocapsules for multimodal imaging-guided stimuli-responsive chemophototherapy(Reprinted with permission from Copyright(2022)American Chemical Society).[91]
4.3. Photoelectric properties of NIR responsive composites
The adsorption of UC materials in terms of photoelectric properties is a promising path for improving solar energy harvesting.[92]Semiconductors are the primary components of solar cells. Photoactivation can be greatly improved if longerwavelength light sources such as IR light can be used for activation. Since the development of UC materials, their applicability in improving the optical frequency response range of semiconductors has become a popular research topic. For example, the limited efficiency of Si-based solar cells is theoretically 33.25%; when combined with UC materials, the value will be 40% by nonconcentrated illumination.[93,94]At present, the highest record of photoelectric conversion efficiency certified by the crystalline silicon cell laboratory is 26.7%, while the efficiency of commercial cells is generally approximately 20%. Owing to the maturity and solidification of technology, the improvement in battery efficiency has been very slow, approximately 0.5% every year. In the past ten years, perovskite has been demonstrated to be an optoelectronic material with significantly advantageous characteristics with excellent performance in the field of solar cells.The photoelectric conversion efficiency of perovskite cells has rapidly increased from 3.8%(2009)[95]to 25.5%in 2021.[96]In terms of electroluminescence, the electro-optical conversion efficiency of green light emission rapidly increased from 0.12% in 2015 to 23.4%.[97]The combination of perovskites and rare earth ions provides new opportunities for expanding its optical properties and functional applicability.[83]As shown in Fig. 4, because of the LSPR-enhanced UCL, UCNPs may widen the response range of perovskite solar cells(PSCs)to the NIR region and boost the Vis light reabsorption of PSCs owing to the scattering and reflection effect,thus generating greater photocurrent in PSCs. Furthermore, UCNPs enhance the perovskite film by efficiently filling the holes and gaps at the grain boundary and reducing the perovskite surface defects, which results in reduced carrier recombination and hence effectively improves PSC device performance.[81]
In summary, NIR-responsive materials can be used to achieve the conversion of low-energy photons to high-energy photons, thereby effectively regulating the wavelength of incident light, triggering photocatalytic reactions, or utilizing light energy. As the QY of UC materials has improved, the development of semiconductor materials,maturity of composite preparation technology,and dynamics of ET efficiency between composite materials have been widely studied. Findings in these fields improve the scope for expanding the spectral response,improving quantum efficiency,and realizing the widespread application of photocatalytic technology. This novel composite material will improve the performance and utility of semiconductors and UC materials in photocatalytic,biomedical,and photovoltaic cell applications.In addition,the commercial application of photocatalytic materials is very demanding in terms of maintaining a broad spectral response and meeting the requirements of high quantum efficiency,high stability, low cost, and simple preparation methods. Therefore,more efforts are needed to realize the application of photocatalysis with UC materials.
Acknowledgments
Project supported by the Interdisciplinary Research Team of Jilin University (Grant No. 10183JXTD202002) and the National Natural Science Foundation of China (Grant Nos.51772121 and 12174150).
相关关键词: photocatalysis